
专家简介

陈军 教授
天津医科大学总医院肺部肿瘤外科主任
博士,教授,主任医师,博士生导师
天津市肺癌研究所所长
天津市胸部肿瘤中心主任
天津市特聘教授,天津市津门英才
天津市“131”第一层次人选,教育新世纪优秀人才
中国老年保健协会肺癌专业委员会主任委员
中国医促会肺癌预防与控制分会副主任委员
中国微循环学会转化医学专业委员会副主任委员
欧美同学会-中国留学人员联谊会医师协会转化医学分会副主任委员
中国医促会胸外科分会常委
中国医师协会胸外科分会委员、中国抗癌学会肿瘤转移分会委员、中国抗癌学会肺癌专委会委员
《中国肺癌杂志》副主编,《Thoracic Cancer》编委
间质上皮细胞转化因子(MET)作为非小细胞肺癌(NSCLC)的关键驱动基因之一,其异常形式多样,包括MET 14外显子跳跃突变、MET扩增、MET蛋白过表达、MET融合及MET活化突变等。不同异常类型的分子特征、检测方法及治疗策略各有差异。近年来,随着对MET异常分子机制的深入研究和多款靶向药物的获批,针对这一靶点的临床诊疗策略日益清晰和精准。本文基于最新循证医学证据,对MET异常NSCLC的检测与治疗进行系统性梳理,旨在为临床实践提供参考。
1. MET异常的概述与流行病学
MET基因位于7号染色体,全长约125kb,包含21个外显子和20个内含子,其编码的蛋白c-Met为跨膜受体酪氨酸激酶,由胞外的SEMA、PSI、IPT1-4结构域和胞内的JM、TK、MDS结构域组成。当肝细胞生长因子(HGF)与c-Met结合后,会诱导c-Met二聚化及自身磷酸化,激活MAPK、PI3K/Akt、STAT3等下游通路,调控细胞增殖、迁移、侵袭及血管生成,其异常激活可通过多种机制驱动肿瘤发生发展[1-2],主要包括:
1.1 MET 14外显子跳跃突变(MET ex14跳跃突变)
MET ex14跳跃突变是MET异常中最经典的激活形式之一,该突变会导致MET蛋白JM结构域缺失,进而引发MET蛋白无法与泛素化蛋白结合,影响MET蛋白的正常降解,进而持续激活下游通路,促进肿瘤的发生发展[3]。MET ex14跳跃突变在中国人群中的发生率为0.9%-2.0%[4-9],不同病理类型上,MET ex14跳跃突变在肺腺癌中发生率约为3%[10],肺鳞癌为1%-2%[11-12],肺肉瘤样癌中可达13%-22%,且多见于老年患者[13-16]。此类患者肿瘤侵袭性强,对传统化疗及免疫治疗反应不佳[8,17-21]。
1.2 MET扩增
MET扩增是指MET基因在肿瘤细胞中的拷贝数异常增加。原发性MET扩增在NSCLC中发生率较低(1%-5%)[17],但它是EGFR-TKIs耐药的重要机制。在一/二代EGFR-TKI耐药后发生率为5%-22%[22-23],第三代EGFR-TKI奥希替尼耐药后发生率为5%-50%[24]。它也是ALK-TKIs的耐药机制之一[25]。
1.3 MET蛋白过表达
MET蛋白过表达可由MET基因扩增、转录上调、缺氧、炎症因子等多种因素引起[26]。其在NSCLC中的发生率报道差异较大,中国人群为17.5%-63.7%[27-29]。在EGFR-TKI经治的EGFR突变患者中,发生率约为30.4%-37.0%,EGFR野生型MET蛋白过表达的发生率约为44.4%[30-31]。随着靶向MET的抗体药物偶联物(ADC)问世,其临床意义日益凸显。
1.4 其他类型
包括MET基因融合(发生率0.26%-0.5%)和激酶区点突变等,虽较为罕见,但同样是潜在的治疗靶点[32]。
2. MET异常的检测
准确检测MET异常是实施精准治疗的前提。当前国内外主要指南(如NCCN、CSCO等)均推荐对晚期NSCLC患者进行MET异常检测[33-34]。
检测必要性:MET ex14跳跃突变已成为晚期NSCLC的必检项目。同时,鉴于MET扩增及过表达在原发性驱动和EGFR-TKI耐药中的关键作用,以及相关靶向药物(包括MET-TKI和MET-ADC)的获批,推荐对初治及EGFR-TKI耐药患者常规进行MET扩增和蛋白过表达检测[33-37,43]。
检测方法:
2.1 MET ex14跳跃突变检测
推荐使用实时定量PCR(RT-qPCR)、DNA二代测序(NGS)或RNA NGS进行检测。DNA-NGS通量高,但需确保探针覆盖关键区域;RNA-NGS可直接检测异常剪接的mRNA,可能具有更高灵敏度,但需优质样本。临床中可根据条件选择或互补验证。
2.2 MET基因扩增检测
荧光原位杂交(FISH)是检测的金标准。常用Cappuzzo标准(GCN≥5)或UCCC标准(MET/CEP7比值≥2.0)进行判读。DNA-NGS检测与FISH的一致性正在提高,但其作为独立检测标准仍需进一步验证。如客观条件不支持进行二次活检的患者可使用液体活检作为补充[17,44-47]。
2.3 MET蛋白过表达检测
免疫组织化学(IHC)是标准检测方法。检测结果的标准化判读至关重要,目前普遍采用“Clinical Score”判读标准,即结合染色强度和阳性肿瘤细胞比例进行综合评分。多项关键临床研究将“≥50%的肿瘤细胞呈现中至强染色(2+/3+)”作为潜在获益的阈值。美国FDA已批准VENTANA MET(SP44)检测作为伴随诊断,其定义的“高表达”即为≥50%肿瘤细胞强染色[38,48,49]。
3. MET异常NSCLC的治疗进展
3.1 MET ex14跳跃突变的治疗
目前,已有5款高选择性MET-TKI在国内获批用于治疗携带MET ex14跳跃突变的局部晚期或转移性NSCLC患者,显著改善了患者预后(表1)[20,50-56]。

赛沃替尼治疗MET ex14跳跃突变的NSCLC的IIIb期临床研究报道的初治人群ORR为62.1%,中位缓解持续时间(duration of remission, DOR)为12.5个月,中位PFS为13.7个月,中位OS为28.3个月;经治人群ORR为39.2%,中位DOR为11.1个月,中位PFS为11个月,中位OS为25.3个月。
谷美替尼GLORY研究是一项单臂、多中心、II期研究,评估了谷美替尼治疗MET ex14跳跃突变的局部晚期或转移性NSCLC的有效性和安全性。总体受试者的ORR为66%,其中初治患者与既往经治患者的ORR分别为71%和60%;中位PFS为8.5个月,其中初治患者为11.7个月,既往经治患者为7.6个月;中位OS为19.4个月,其中初治患者为25.4个月,既往经治患者为16.3个月;基线有脑转移的13例患者中,ORR为85%(11/13),其中5例患者的脑转移病灶被选为靶病灶并在治疗后测量,该5例患者均观察到颅内PR,颅内ORR为100%。
伯瑞替尼KUNPENG研究是一项针对局部晚期或转移性MET异常的NSCLC患者的II期研究,MET外显子14跳突队列共纳入52例NSCLC患者,经BIRC评估的总体ORR为75.0%,疾病控制率(disease control rate, DCR)为96.2%,中位DOR为16.5个月,中位PFS为14.3个月,中位OS为20.3个月,3年OS率为35.1%。亚组分析显示,初治患者ORR为77.1%,既往经治患者ORR为70.6%。
特泊替尼VISION研究显示,总体患者人群中的ORR为51.4%,中位DOR为18个月,中位PFS为11.2个月,中位OS为19.6个月。
卡马替尼GEOMETRY mono-1研究结果显示,在初治患者队列中ORR为68%,中位DOR为16.6个月,中位PFS为12.5个月,中位OS为21.4个月;既往经治患者的ORR为44%,中位DOR为9.7个月,中位PFS为5.5个月,中位OS为16.8个月。
此外,也有研究积极探索非TKIs类药物治疗MET 14跳突NSCLC的效果。埃万妥单抗CHRYSALIS[57] I期研究中,MET ex14队列的总ORR为33%,中位DOR为11.2个月。
3.2 MET扩增的治疗
MET扩增的治疗需区分原发性扩增与EGFR-TKI耐药后继发性扩增。
3.2.1 原发性MET扩增:现有临床研究数据(表2)提示MET-TKIs可能为此类患者带来获益[56-60]。伯瑞替尼在KUNPENG研究的扩增队列中显示出前景,在MET基因拷贝数≥6的患者中,总体ORR为48.8%,中位PFS为7.4个月,并基于此数据获NMPA附条件批准用于治疗MET扩增NSCLC。卡马替尼的研究提示,其疗效与扩增程度相关,在高水平扩增(基因拷贝数≥10)的患者中观察到更好的缓解率。
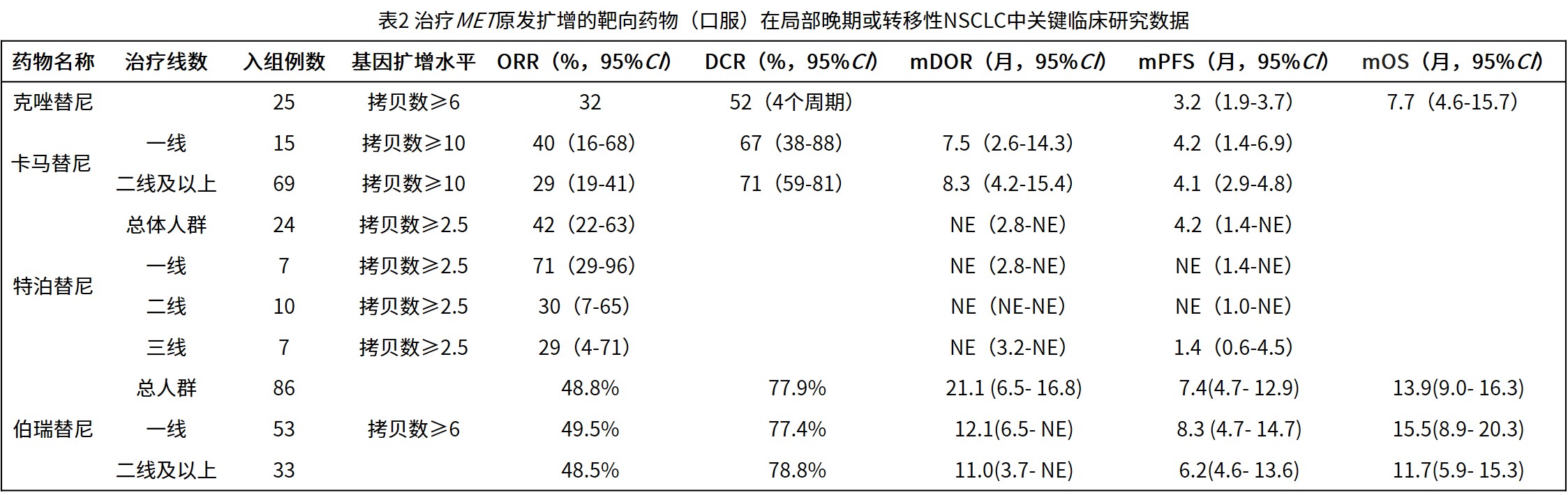
3.2.2 EGFR-TKI耐药后继发性MET扩增:是当前临床实践中的常见且重要场景。采用EGFR-TKI联合MET-TKI的双靶向策略,可有效克服EGFR-TKI耐药后旁路激活(表3)[36-42,61,65-66]。
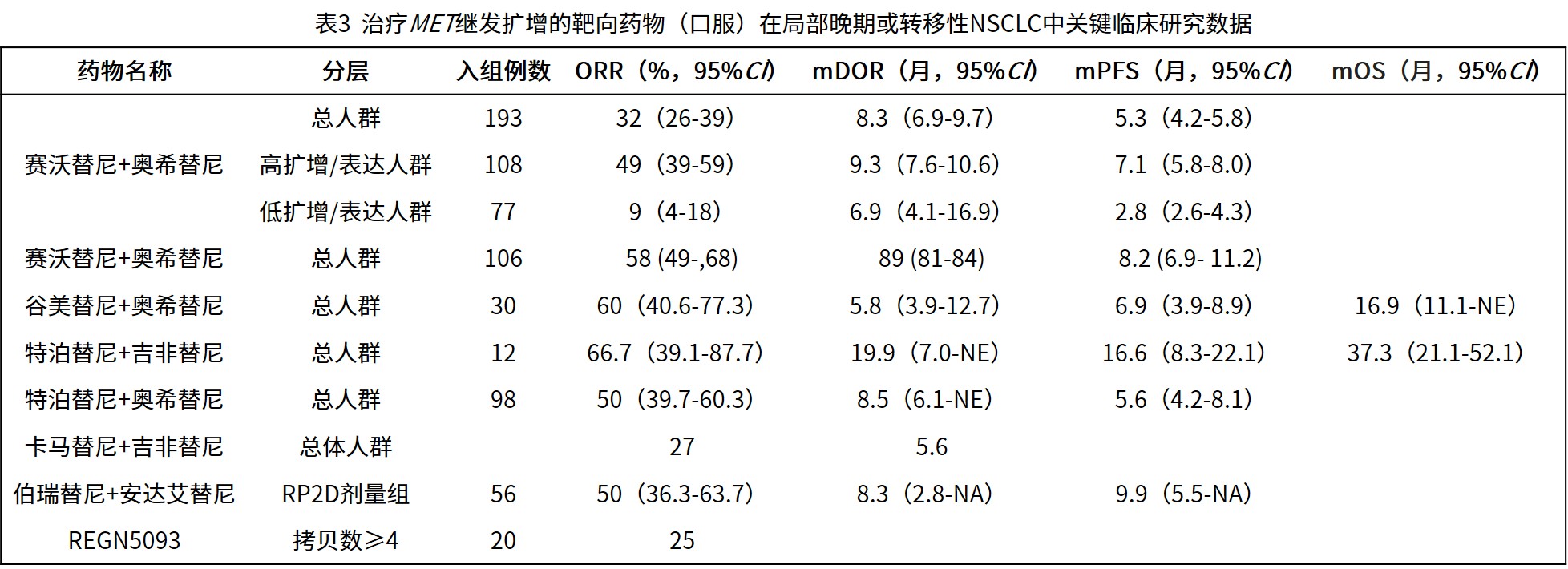
赛沃替尼联合奥希替尼:SAVANNAH研究揭示,疗效与MET扩增/过表达水平密切相关。在MET高水平扩增或过表达(GCN≥10和/或IHC 90% 3+)的患者亚组中,联合治疗的ORR达49%,中位PFS为7.1个月;而在低水平(非GCN≥10和/或IHC 90% 3+)患者中获益有限,ORR仅9%,中位PFS为2.8个月。随后的III期SACHI研究评估了该联合方案对比化疗用于晚期NSCLC一线EGFR-TKI进展后伴MET扩增(一/二代EGFR-TKI经治:FISH检测MET GCN≥5或MET/CEP7≥2.0,且T790M阴性;三代EGFR-TKI经治:MET GCN≥10)的疗效和安全性,结果显示,联合组在ORR、DCR方面均显著优于化疗组,且能够延长患者的PFS。基于此数据,赛沃替尼获NMPA批准用于EGFR基因突变阳性,经EGFR-TKI治疗后进展的伴MET扩增的局部晚期或转移性非鳞状NSCLC患者。
谷美替尼联合奥希替尼:SCC244-203研究评估了谷美替尼联合奥希替尼治疗第一、二、三代EGFR-TKIs耐药后MET扩增NSCLC患者的有效性和安全性。在整体人群中,ORR达到60%,DCR达到90%,中位PFS达6.9个月,中位OS达16.9个月。
特泊替尼联合吉非替尼:INSIGHT研究结果显示,与化疗相比,联合组改善了EGFR-TKIs耐药后MET扩增NSCLC患者的PFS、OS、ORR和DOR:特泊替尼联合吉非替尼组和化疗组的中位PFS分别为16.6和4.2个月(HR=0.13),中位OS分别为37.3和13.1个月(HR=0.10),ORR分别为66.7%和42.9%,中位DOR分别为19.9和2.8个月。
特泊替尼联合奥希替尼:INSIGHT 2的主要分析[41]显示,在伴有MET扩增且一线奥希替尼进展的EGFR突变NSCLC患者中,特泊替尼联合奥希替尼治疗的ORR为50%,中位DOR为8.5个月。
卡马替尼联合吉非替尼:一项Ib/II期研究评估了卡马替尼联合吉非替尼治疗EGFR-TKIs治疗失败并伴有MET扩增/过表达的NSCLC患者的有效性和安全性。II期研究的ORR为27%,中位DOR为5.6个月。在高MET扩增肿瘤的患者中,MET活性增加,MET基因GCN≥6的患者的II期ORR为47%。
伯瑞替尼联合PLB1004:KYLIN-1是一项Ib/II期研究,旨在评估伯瑞替尼联合PLB1004(安达艾替尼)治疗EGFR-TKIs耐药后伴MET扩增/过表达NSCLC患者的有效性和安全性。该研究在RP2D剂量下共入组56例患者,确认的ORR为50.0%。DCR为89.3%,mPFS为9.9个月。经一代/二代EGFR-TKI治疗后进展患者,DCR为87.8%,经三代EGFR-TKI治疗后进展患者,DCR为89.5%,mPFS为9.6个月。
也有研究积极探索非TKIs类药物治疗MET扩增NSCLC的效果。REGN5093是一种新型的双特异性抗体药物,同时靶向MET和EGFR。一项I/II期研究评估了REGN5093单药治疗MET异常晚期NSCLC患者的有效性,研究发现,MET扩增患者队列的ORR为25%。
3.3 MET蛋白过表达的治疗
MET蛋白过表达的治疗根据其背景分为两类:
EGFR-TKI耐药后MET蛋白过表达:研究提示,IHC检测为高表达(如≥50%肿瘤细胞强染色3+)的患者可能从EGFR/MET双靶联合中获益。INSIGHT、TATTON等研究为此提供了依据[48,49]。此外,MET-ADC药物Telisotuzumab vedotin(Teliso-V)联合厄洛替尼在经治患者中显示出抗肿瘤活性,尤其在MET高表达亚组。埃万妥单抗联合拉泽替尼在奥希替尼耐药、EGFR/MET高表达的NSCLC中也展现出潜在获益[68]。
驱动基因阴性MET蛋白过表达:对于不伴有其他明确驱动基因的MET过表达NSCLC,治疗选择正在拓展。谷美替尼单药在此类患者中ORR为37.5%,中位OS为17个月[62];其联合化疗的研究也显示出积极信号:在驱动基因阴性的MET蛋白表达2+或3+(IHC≥50% 2+且<50% 3+或IHC ≥50% 3+)二线患者中,联合方案的整体ORR为 34.8%,其中IHC 3+亚组为60%,DCR在整体、IHC 3+和IHC 2+亚组分别为95.7%、100%和94.4%[69]。LUMINOSITY研究则证实了Teliso-V单药在EGFR野生型、MET高表达非鳞NSCLC二线治疗中的疗效,并基于此获FDA加速批准,用于治疗经治的EGFR野生型、MET高表达(IHC 3+≥50%)非鳞状NSCLC患者[70]。但Teliso-V目前还未在国内获批上市。
4. 未来展望
尽管MET异常NSCLC治疗取得了长足进步,但未来仍面临诸多挑战与机遇。
检测标准化:统一MET扩增的NGS判读标准、优化MET蛋白IHC的检测与判读阈值,是确保精准治疗的前提。液体活检(如ctDNA)在动态监测耐药中的应用值得深化。未来可能需结合多种生物标志物(如MET蛋白表达、特定突变等)进行综合评估,以实现更精准的疗效预测。
优化现有治疗与克服耐药:对于MET扩增,需要更多III期研究确立标准治疗方案,并探索MET-TKIs与其他靶向药的联合策略。此外,克服MET-TKIs的获得性耐药是关键挑战,耐药机制包括靶点依赖性突变(如MET TKD突变D1228N/Y1248H等)和旁路激活。探索针对这些耐药突变的新一代药物,或采用ADC、双特异性抗体等不同作用机制的药物,是未来的研究方向。
拓展MET过表达治疗适应症:MET-ADC(如Teliso-V)的FDA获批以及谷美替尼数据的公布为驱动基因阴性MET蛋白过表达患者提供了新选择。未来还需要更大规模的II和III期临床试验来进一步验证疗效和安全性。
探索罕见MET异常及新治疗场景:对于MET融合、KDD(激酶结构域复制)等罕见异常,需积累更多临床数据明确其最佳治疗策略[71-73]。此外,MET-TKIs在可手术早期NSCLC中的辅助或新辅助治疗价值,正在多项临床试验中进行探索,初步的个案报告[74-77]显示了其诱导肿瘤缩小的潜力,有望为早期患者带来新获益。
总结
当前,MET异常NSCLC的诊疗已进入分子分型指导下的精准时代。在治疗上,针对MET ex14跳跃突变,多种高效MET-TKI可供选择;针对MET扩增,靶向治疗已成为标准策略;而对于MET蛋白过表达,MET-TKI和ADC药物治疗有了新的突破。未来,通过检测技术的标准化、治疗策略的优化、耐药机制的攻克以及治疗前移的探索,有望为更多MET异常NSCLC患者带来生存获益。
【参考文献】
References
[1]Friedlaender A, Perol M, Banna GL, et al. Oncogenic alterations in advanced NSCLC: a molecular super-highway. Biomark Res, 2024, 12(1): 24. doi: 10.1186/s40364-024-00566-0
[2]Friedlaender A, Drilon A, Banna GL, et al. The METeoric rise of MET in lung cancer[J]. Cancer, 2020, 126(22):4826-4837. DOI: 10.1002/cncr.33159.
[3]Santarpia M, Massafra M, Gebbia V, et al. A narrative review of MET inhibitors in non-small cell lung cancer with MET exon 14 skipping mutations[J]. Transl Lung Cancer Res, 2021, 10(3):1536-1556. DOI: 10.21037/tlcr-20-1113.
[4]Liu SY, Gou LY, Li AN, et al. The Unique Characteristics of MET Exon 14 Mutation in Chinese Patients with NSCLC[J]. J Thorac Oncol, 2016, 11(9):1503-1510. DOI: 10.1016/j.jtho.2016.05.016.
[5]Qiu T, Li W, Zhang T, et al. Distinct MET Protein Localization Associated With MET Exon 14 Mutation Types in Patients With Non-small-cell Lung Cancer[J]. Clin Lung Cancer, 2018, 19(4):e391-e398. DOI: 10.1016/j.cllc.2017.12.006.
[6]Xu Z, Li H, Dong Y, et al. Incidence and PD-L1 Expression of MET 14 Skipping in Chinese Population: A Non-Selective NSCLC Cohort Study Using RNA-Based Sequencing[J]. Onco Targets Ther, 2020, 13:6245-6253. DOI: 10.2147/OTT.S241231.
[7]Zheng D, Wang R, Ye T, et al. MET exon 14 skipping defines a unique molecular class of non-small cell lung cancer[J]. Oncotarget, 2016, 7(27):41691-41702. DOI: 10.18632/oncotarget.9541.
[8]Tong JH, Yeung SF, Chan AW, et al. MET Amplification and Exon 14 Splice Site Mutation Define Unique Molecular Subgroups of Non-Small Cell Lung Carcinoma with Poor Prognosis[J]. Clin Cancer Res, 2016, 22(12):3048-3056. DOI: 10.1158/1078-0432.CCR-15-2061.
[9]Gow CH, Hsieh MS, Wu SG, et al. A comprehensive analysis of clinical outcomes in lung cancer patients harboring a MET exon 14 skipping mutation compared to other driver mutations in an East Asian population[J]. Lung Cancer, 2017, 103:82-89. DOI: 10.1016/j.lungcan.2016.12.001.
[10]Liang H, Wang M. MET Oncogene in Non-Small Cell Lung Cancer: Mechanism of MET Dysregulation and Agents Targeting the HGF/c-Met Axis[J]. Onco Targets Ther, 2020, 13:2491-2510. DOI: 10.2147/OTT.S231257.
[11]Schrock AB, Frampton GM, Suh J, et al. Characterization of 298 Patients with Lung Cancer Harboring MET Exon 14 Skipping Alterations[J]. J Thorac Oncol, 2016, 11(9):1493-1502. DOI: 10.1016/j.jtho.2016.06.004.
[12]Lam VK, Tran HT, Banks KC, et al. Targeted Tissue and Cell-Free Tumor DNA Sequencing of Advanced Lung Squamous-Cell Carcinoma Reveals Clinically Significant Prevalence of Actionable Alterations[J]. Clin Lung Cancer, 2019, 20(1):30-36.e3. DOI: 10.1016/j.cllc.2018.08.020.
[13]Vuong HG, Ho A, Altibi A, et al. Clinicopathological implications of MET exon 14 mutations in non-small cell lung cancer - A systematic review and meta-analysis[J]. Lung Cancer, 2018, 123:76-82. DOI: 10.1016/j.lungcan.2018.07.006.
[14]Liu X, Jia Y, Stoopler MB, et al. Next-Generation Sequencing of Pulmonary Sarcomatoid Carcinoma Reveals High Frequency of Actionable MET Gene Mutations[J]. J Clin Oncol, 2016, 34(8):794-802. DOI: 10.1200/JCO.2015.62.0674.
[15]Fujino T, Suda K, Mitsudomi T. Lung Cancer with MET exon 14 Skipping Mutation: Genetic Feature, Current Treatments, and Future Challenges[J]. Lung Cancer (Auckl), 2021, 12:35-50. DOI: 10.2147/LCTT.S269307.
[16]Awad MM, Oxnard GR, Jackman DM, et al. MET Exon 14 Mutations in Non-Small-Cell Lung Cancer Are Associated With Advanced Age and Stage-Dependent MET Genomic Amplification and c-Met Overexpression[J]. J Clin Oncol, 2016, 34(7):721-730. DOI: 10.1200/JCO.2015.63.4600.
[17]Guo R, Luo J, Chang J, et al. MET-dependent solid tumours - molecular diagnosis and targeted therapy[J]. Nat Rev Clin Oncol, 2020, 17(9):569-587. DOI: 10.1038/s41571-020-0377-z.
[18]Drilon A, Clark JW, Weiss J, et al. Antitumor activity of crizotinib in lung cancers harboring a MET exon 14 alteration[J]. Nat Med, 2020, 26(1):47-51. DOI: 10.1038/s41591-019-0716-8.
[19]Paik PK, Felip E, Veillon R, et al. Tepotinib in Non-Small-Cell Lung Cancer with MET Exon 14 Skipping Mutations[J]. N Engl J Med, 2020, 383(10):931-943. DOI: 10.1056/NEJMoa2004407.
[20]Yu Y, Zhou J, Li X, et al. Gumarontinib in patients with non-small-cell lung cancer harbouring MET exon 14 skipping mutations: a multicentre, single-arm, open-label, phase 1b/2 trial[J]. EClinicalMedicine, 2023, 59:101952. DOI: 10.1016/j.eclinm.2023.101952.
[21]Lung Cancer Specialty Committee of Chinese Elderly Health Care Association. Expert consensus on targeted therapy of NSCLC with MET exon 14 skipping mutation. Zhongguo Feiai Zazhi, 2023, 26(6): 416-428. [中国老年保健协会肺癌专业委员会. MET 14外显子跳跃突变NSCLC靶向治疗专家共识. 中国肺癌杂志, 2023, 26(6): 416-428.] doi: 10.3779/j.issn.1009-3419.2023.102.19.
[22]Westover D, Zugazagoitia J, Cho BC, et al. Mechanisms of acquired resistance to first- and second-generation EGFR tyrosine kinase inhibitors[J]. Ann Oncol, 2018, 29(suppl_1):i10-i19. DOI: 10.1093/annonc/mdx703.
[23]Matikas A, Mistriotis D, Georgoulias V, et al. Current and Future Approaches in the Management of Non-Small-Cell Lung Cancer Patients With Resistance to EGFR TKIs[J]. Clin Lung Cancer, 2015, 16(4):252-261. DOI: 10.1016/j.cllc.2014.12.013.
[24]Leonetti A, Sharma S, Minari R, et al. Resistance mechanisms to osimertinib in EGFR-mutated non-small cell lung cancer[J]. Br J Cancer, 2019, 121(9):725-737. DOI: 10.1038/s41416-019-0573-8.
[25]Dagogo-Jack I, Yoda S, Lennerz JK, et al. MET Alterations Are a Recurring and Actionable Resistance Mechanism in ALK-Positive Lung Cancer[J]. Clin Cancer Res, 2020, 26(11):2535-2545. DOI: 10.1158/1078-0432.CCR-19-3906.
[26]Yu X, Xu Y, Fan Y. Progress of c-MET Signaling Pathway and TKIs in Non-small Cell Lung Cancer. Zhongguo Fei Ai Za Zhi. 2017 Apr 20;20(4):287-292. [俞晓晴,徐艳珺,范云,等.c-MET通路和抑制剂在非小细胞肺癌中的研究进展[J].中国肺癌杂志, 2017, 20(4):6.] doi: 10.3779/j.issn.1009-3419.2017.04.10.
[27]Park S, Choi YL, Sung CO, et al. High MET copy number and MET overexpression: poor outcome in non-small cell lung cancer patients[J]. Histol Histopathol, 2012, 27(2):197-207. DOI: 10.14670/HH-27.197.
[28]Lv H, Shan B, Tian Z, et al. Soluble c-Met is a reliable and sensitive marker to detect c-Met expression level in lung cancer[J]. Biomed Res Int, 2015, 2015:626578. DOI: 10.1155/2015/626578.
[29]LI X, Chen Z, XI Y, et al. Correlation between expression of C-met and epidermal growth factor receptor-tyrosine kinase inhibitor resistance in lung adenocarcinoma. Cancer Research and Clinic. 2018:1-6.[李雄峰,陈振文,郗彦凤,等.肺腺癌中C—met表达与表皮生长因子受体-酪氨酸激酶抑Stain耐药的相关性[J].肿瘤研究与临床, 2018, 30(1):6.] doi: 10.3760/cma. j. issn.1006‑9801.2018.01.001.
[30] Kim IH, Lee IH, Lee JE, et al. Clinical Significance of C-MET Overexpression and Epidermal Growth Factor Receptor Mutation in Platinum-Based Adjuvant Chemotherapy Outcome in Surgically Resected Lung Adenocarcinoma. Ann Surg Oncol. 2017;24(3):770-777. DOI: 10.1245/s10434-016-5599-z
[31]Watermann I, Schmitt B, Stellmacher F, et al. Improved diagnostics targeting c-MET in non-small cell lung cancer: expression, amplification and activation?[J]. Diagn Pathol, 2015, 10:130. DOI: 10.1186/s13000-015-0362-5.
[32]Zhuo M, Liang Z, Yi Y, et al. Analysis of MET kinase domain rearrangement in NSCLC[J]. Lung Cancer, 2020, 145:140-143. DOI: 10.1016/j.lungcan.2020.04.040.
[33]NCCN guidelines-Non-small cell lung cancer Version 8.2024. https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1450.
[34]Working Committee of the Chinese Society of Clinical Oncology Guidelines. Chinese Society of Clinical Oncology (CSCO) Guidelines for the Diagnosis and Treatment of Non Small Cell Lung Cancer 2024. Beijing: People's Health Publishing House, 2024. [中国临床肿瘤学会指南工作委员会. 中国临床肿瘤学会(CSCO)非小细胞肺癌诊疗指南2024. 北京: 人民卫生出版社, 2024.
[35]Chinese Society of Pathology, Pathology Quality Control Center, Lung Cancer Group of Chinese Medical Association Chinese Society of Oncology, et al. Chinese expert consensus on clinical practice of MET detection in non-small cell lung cancer.Zhonghua bing li xue Zazhi, 2022,51(11):1094-1103. [中华医学会病理学分会,国家病理质控中心,中华医学会肿瘤学分会肺癌学组,等.非小细胞肺癌MET临床检测中国专家共识[J].中华病理学杂志, 2022,51(11):1094-1103.] doi:10.3760/cma.j.cn112151-20220606-00491.
[36]Ahn MJ, Mendoza M, Pavlakis N, et al. Asian Thoracic Oncology Research Group (ATORG) Expert Consensus Statement on MET Alterations in NSCLC: Diagnostic and Therapeutic Considerations[J]. Clin Lung Cancer, 2022, 23(8):670-685. DOI: 10.1016/j.cllc.2022.07.012.
[37]中国医疗保健国际交流促进会肿瘤内科学分会, 中国医师协会肿瘤医师分会. Ⅳ期原发性肺癌中国治疗指南(2024版)[J]. 中华肿瘤杂志, 2024, 46(07):595-636. DOI: 10.3760/cma.j.cn112152-20240311-00104.
[38]Ahn M, Marinis FD, Bonanno L, et al. EP08.02-140 MET Biomarker-based Preliminary Efficacy Analysis in SAVANNAH: savolitinib+osimertinib in EGFRm NSCLC Post-Osimertinib[J]. Journal of Thoracic Oncology, 2022 . DOI: 10.1016/j.jtho.2022.07.823.
[39]Yanmin Yu,N. Yang,Y. Zhang, et al. SCC244 plus osimertinib in patients with stage IIIB/IIIC or IV, EGFR TKI resistant EGFR-mutant NSCLC harboring MET amplification. ESMO ASIA 2022,305MO.DOI:10.1016/j.annonc.2022.10.334
[40]Ohara R, Liam CK, Ahmad AR, et al. PPD02.02 Tepotinib + Gefitinib in Patients with EGFR-Mutant NSCLC with MET Amplification (METamp): Final Analysis of INSIGHT[J]. Journal of Thoracic Oncology, 2023 . DOI: 10.1016/j.jtho.2022.09.020.
[41]T.M. Kim, V. Guarneri, V. P. Jye, et al. Tepotinib + Osimertinib in EGFR-mutant NSCLC with MET Amplification Following 1L Osimertinib: INSIGHT 2 Primary Analysis. 2023 WCLC OA21.05. DOI:10.1016/j.jtho.2023.09.106
[42]Wu YL, Zhang L, Kim DW, et al. Phase Ib/II Study of Capmatinib (INC280) Plus Gefitinib After Failure of Epidermal Growth Factor Receptor (EGFR) Inhibitor Therapy in Patients With EGFR-Mutated, MET Factor-Dysregulated Non-Small-Cell Lung Cancer[J]. J Clin Oncol, 2018, 36(31):3101-3109. DOI: 10.1200/JCO.2018.77.7326.
[43]MPCG of Tumor, Pathology Committee of Chinese Anti-Cancer Association. Chinese expert consensus on MET immunohistochemistry detection and interpretation standards for non-small cell lung cancer (2023 version). Zhonghua bing li xue za zhi= Chinese journal of pathology. 2023 Nov 8;52(11):1090-7. [MET免疫组织化学标准化判读专家委员会,中国抗癌协会肿瘤病理专业委员会分子病理协作组. 非小细胞肺癌MET免疫组织化学检测和判读标准中国专家共识(2023版)[J]. 中华病理学杂志,2023,52(11):1090-1097.] doi:10.3760/cma.j.cn112151-20230626-00423.
[44] 武国栋,谷文光,袁明明,等. 胸腔积液在肺腺癌基因检测中的应用研究[J]. 中华肿瘤杂志,2022,44(08):873-876. DOI:10.3760/cma.j.cn112152-20210308-00204.
[45]Noonan SA, Berry L, Lu X, et al. Identifying the Appropriate FISH Criteria for Defining MET Copy Number-Driven Lung Adenocarcinoma through Oncogene Overlap Analysis[J]. J Thorac Oncol, 2016, 11(8):1293-1304. DOI: 10.1016/j.jtho.2016.04.033.
[46]Xiang C, Lv X, Chen K, et al. Unraveling the Significance of MET Focal Amplification in Lung Cancer: Integrative NGS, FISH, and IHC Investigation. Mod Pathol. 2024;37(4):100451.
[47]Zheng Q, Lin X, Qi W, et al. NGS and FISH for MET amplification detection in EGFR TKI resistant non-small cell lung cancer (NSCLC) patients: A prospective, multicenter study in China. Lung Cancer. 2024;194:107897.
[48]Wu YL, Cheng Y, Zhou J, et al. Tepotinib plus gefitinib in patients with EGFR-mutant non-small-cell lung cancer with MET overexpression or MET amplification and acquired resistance to previous EGFR inhibitor (INSIGHT study): an open-label, phase 1b/2, multicentre, randomised trial[J]. Lancet Respir Med, 2020, 8(11):1132-1143. DOI: 10.1016/S2213-2600(20)30154-5.
[49]Hartmaier RJ, Markovets AA, Ahn MJ, et al. Osimertinib + Savolitinib to Overcome Acquired MET-Mediated Resistance in Epidermal Growth Factor Receptor-Mutated, MET-Amplified Non-Small Cell Lung Cancer: TATTON[J]. Cancer Discov, 2023, 13(1):98-113. DOI: 10.1158/2159-8290.CD-22-0586.
[50]Yu Y, Guo Q, Zhang Y, et al. Savolitinib in patients in China with locally advanced or metastatic treatment-naive non-small-cell lung cancer harbouring MET exon 14 skipping mutations: results from a single-arm, multicohort, multicentre, open-label, phase 3b confirmatory study[J]. Lancet Respir Med, 2024, 12(12):958-966. DOI: 10.1016/S2213-2600(24)00211-X.
[51]Yu Y, Zhou J, Li X, et al. Efficacy and safety outcomes of Phase II study of SCC244 in NSCLC patients harboring MET exon 14 skipping (METex14) mutations (GLORY study): long-term follow-up analysis. Annals of Oncology (2024) 35 (suppl_4): S1632-S1678. 10.1016/annonc/annonc1698
[52]Jin-Ji Yang, Yan Zhang, Lin Wu, et al. Efficacy and safety of vebreltinib in patients with advanced NSCLC harboring MET exon 14-skipping: Results of 2.5-year follow-up in KUNPENG[J]. Journal of Clinical Oncology, 2024, 42(16)_suppl, DOI: 10.1200/JCO.2024.42.16_suppl.8557.
[53]Zhang Y, Zhang H, Wang H, et al. Use of savolitinib as neoadjuvant therapy for non-small cell lung cancer patient with MET exon 14 skipping alterations: A case report[J]. Front Oncol, 2022, 12:968030. DOI: 10.3389/fonc.2022.968030.
[54] Yongfeng Yu , et al. Final Overall Survival and Long-term Safety Outcomes of Savolitinib in Patients with Locally Advanced or Metastatic NSCLC Harboring MET Exon 14 (METex14) Mutation: An Update from a Phase 3b Study. 2025 ELCC Abstract 80P.
[55]Mazieres J, Paik PK, Garassino MC, et al. Tepotinib Treatment in Patients With MET Exon 14-Skipping Non-Small Cell Lung Cancer: Long-term Follow-up of the VISION Phase 2 Nonrandomized Clinical Trial[J]. JAMA Oncol, 2023, 9(9):1260-1266. DOI: 10.1001/jamaoncol.2023.1962.
[56]Wolf J, Hochmair M, Han JY, et al. Capmatinib in MET exon 14-mutated non-small-cell lung cancer: final results from the open-label, phase 2 GEOMETRY mono-1 trial[J]. Lancet Oncol, 2024, 25(10):1357-1370. DOI: 10.1016/S1470-2045(24)00441-8.
[57] Leighl N, Cho BC, Hiret S, Han J-Y, Lee KH, Llacer Perez C.OA21.04 – Amivantamab in patients with advanced NSCLC and MET exon 14 skipping mutation: results from the CHRYSALIS study. J Thorac Oncol. 2023; 18: S93-4.
[58]Moro-Sibilot D, Cozic N, Pérol M, et al. Crizotinib in c-MET- or ROS1-positive NSCLC: results of the AcSé phase II trial[J]. Ann Oncol, 2019, 30(12):1985-1991. DOI: 10.1093/annonc/mdz407.
[59]Le X, Paz-Ares LG, Meerbeeck JV, et al. Tepotinib in patients (pts) with advanced non-small cell lung cancer (NSCLC) with MET amplification ( MET amp)[J]. JOURNAL OF CLINICAL ONCOLOGY, 2021, 39(15_suppl):9021-9021. DOI: 10.1200/JCO.2021.39.15_suppl.9021.
[60] 伯瑞替尼药物说明书2025年6月版.
[61]B.C. Cho, M. Ahn, T.M. Kim, et al. Early safety, tolerability, and efficacy of REGN5093 in patients (pts) with MET-altered advanced non-small cell lung cancer (aNSCLC) from a first in human (FIH) study[J]. Annals of Oncology (2022) 33 (suppl_7): S448-S554. DOI: 10.1016/annonc/annonc1064
[62]Yu Y, Dong W, Shi Y, et al. A pooled analysis of clinical outcome in driver-gene negative non-small cell lung cancer patients with MET overexpression treated with gumarontinib[J]. Ther Adv Med Oncol, 2024, 16:17588359241264730. DOI: 10.1177/17588359241264730.
[63]Camidge DR, Barlesi F, Goldman JW, et al. Phase Ib Study of Telisotuzumab Vedotin in Combination With Erlotinib in Patients With c-Met Protein-Expressing Non-Small-Cell Lung Cancer[J]. J Clin Oncol, 2023, 41(5):1105-1115. DOI: 10.1200/JCO.22.00739.
[64]Goldman JW, Horinouchi H, Cho BC, et al. Phase 1/1b study of telisotuzumab vedotin (Teliso-V) + osimertinib (Osi), after failure on prior Osi, in patients with advanced, c-Met overexpressing,EGFR-mutated non-small cell lung cancer (NSCLC)[J]. JOURNAL OF CLINICAL ONCOLOGY, 2022, 40(16_suppl):9013-9013. DOI: 10.1200/JCO.2022.40.16_suppl.9013.
[65] .Shun Lu, et al. Savolitinib (Savo) combined with osimertinib (osi) versus chemotherapy (chemo) in EGFR-mutant (EGFRm) and MET-amplification (METamp) advanced NSCLC after disease progression (PD) on EGFR tyrosine kinase inhibitor (TKI): Results from a randomized phase 3 SACHI study. 2025 ASCO Abstract LBA8505.
[66] Fei Zhou, Shengxiang Ren, Guowei Che, Chen Zhou, et al. Vebreltinib plus PLB1004 in EGFR-mutated NSCLC with acquired MET amplification or overexpression after failure on EGFR-TKI treatment: A phase Ib/II study. 2025 ASCO Abstract 8632.
[67]Peng LX, Jie GL, Li AN, et al. MET amplification identified by next-generation sequencing and its clinical relevance for MET inhibitors[J]. Exp Hematol Oncol, 2021, 10(1):52. DOI: 10.1186/s40164-021-00245-y.
[68] Cho BC, Kim DW, Spira AI, Gomez JE, Haura EB, Kim SW, Sanborn RE, Cho EK, Lee KH, Minchom A, Lee JS, Han JY, Nagasaka M, Sabari JK, Ou SI, Lorenzini P, Bauml JM, Curtin JC, Roshak A, Gao G, Xie J, Thayu M, Knoblauch RE, Park K. Amivantamab plus lazertinib in osimertinib-relapsed EGFR-mutant advanced non-small cell lung cancer: a phase 1 trial. Nat Med. 2023 Oct;29(10):2577-2585. doi: 10.1038/s41591-023-02554-7. Epub 2023 Sep 14. PMID: 37710001; PMCID: PMC10579096.
[69] .Yu Y, et al. Glumetinib plus albumin-bound docetaxel (HB1801) in patients with MET overexpression, actionable genetic alteration- (AGA-) negative Non-Small Cell Lung Cancer (Gleam Study). 2025 ESMO ASIA Abstract 989P.
[70] Camidge DR, Bar J, Horinouchi H, et al. Telisotuzumab Vedotin Monotherapy in Patients With Previously Treated c-Met Protein-Overexpressing Advanced Nonsquamous EGFR-Wildtype Non-Small Cell Lung Cancer in the Phase II LUMINOSITY Trial. J Clin Oncol. 2024;42(25):3000-3011. DOI:10.1200/JCO.24.00720.
[71]Kang J, Deng QM, Feng W, et al. Response and acquired resistance to MET inhibitors in de novo MET fusion-positive advanced non-small cell lung cancer[J]. Lung Cancer, 2023, 178:66-74. DOI: 10.1016/j.lungcan.2023.01.017.
[72]Nakazawa S, Pecci F, Odintsov I, et al. Antitumor Activity of Vebreltinib and Characterization of Clinicogenomic Features in Solid Tumors with MET Rearrangements. Cancer Discov. 2025;15(6):1129-1140.
[73]Xu CW, Wang WX, Wang XJ, et al. Abstract 1597: Real-world large-scale study kinase domain duplications across diverse tumor types in Chinese populations[J]. 2019 .Cancer Res (2019) 79 (13_Supplement): 1597. DOI.org/10.1158/1538-7445.AM2019-1597
[84]Zhang Y, Zhang H, Wang H, et al. Use of savolitinib as neoadjuvant therapy for non-small cell lung cancer patient with MET exon 14 skipping alterations: A case report[J]. Front Oncol, 2022, 12:968030. DOI: 10.3389/fonc.2022.968030.
[75]Deng HY, Qiu XM, Zhu DX, et al. The safety and feasibility of preoperative induction therapy of Savolitinib in non-small cell lung cancer patients with MET exon 14 skipping mutation[J]. J Cancer Res Clin Oncol, 2023, 149(8):4623-4628. DOI: 10.1007/s00432-022-04370-x.
[76]Tian J, Lin Z, Chen Y, et al. Dramatic response to neoadjuvant savolitinib in marginally resectable lung adenocarcinoma with MET exon 14 skipping mutation: A case report and literature review[J]. Front Oncol, 2022, 12:1006634. DOI: 10.3389/fonc.2022.1006634.
[77]Fu M, Feng CM, Xia DQ, et al. Neoadjuvant Savolitinib targeted therapy stage IIIA-N2 primary lung adenocarcinoma harboring MET Exon 14 skipping mutation: A case report[J]. Front Oncol, 2022, 12:954886. DOI: 10.3389/fonc.2022.954886.
编辑:Lagertha
审核:陈军教授
来源:肿瘤界